As part of the collaborative project SynergyFuels, 3 professorships from the TUM-Campus Straubing are responsible for the sustainable synthesis of formaldehyde: Professorship Chemical Process Engineering (TUM CTV), Chair of Chemistry of Biogenic Resources (TUM CBR), and Professorship Electrobiotechnology (TUM EBT).
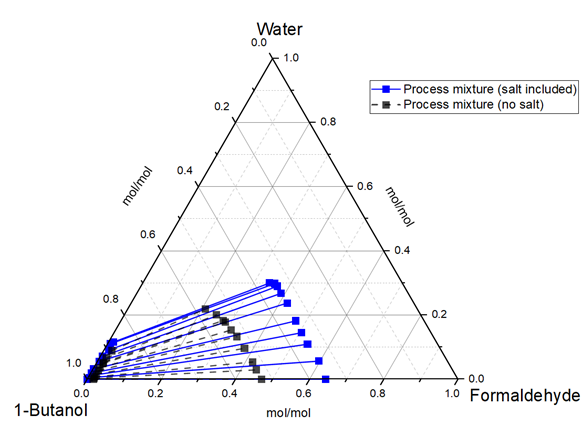
In order to conceptualize the process of synthesizing formaldehyde and the production of feedstocks for the OMBE synthesis, multiple experiments were performed at TUM CTV to measure the vapor-liquid and liquid-liquid equilibria of the process mixture. Given the presence of salts and buffers in the process media, their effects on equilibria were also measured. An apparatus was custom-manufactured to capture the salt effect on the vapor-liquid equilibrium since the existing apparatus was inaccurate in capturing this effect.
An activity model was developed to describe the behavior of the process mixture. This model will then be used to simulate and assess the feasibility of various downstream processes, including but not limited to reactive distillation and liquid-liquid extraction. Additionally, a selection of different adsorbents has been identified and experiments will be performed to determine the feasibility of incorporating an adsorption process.

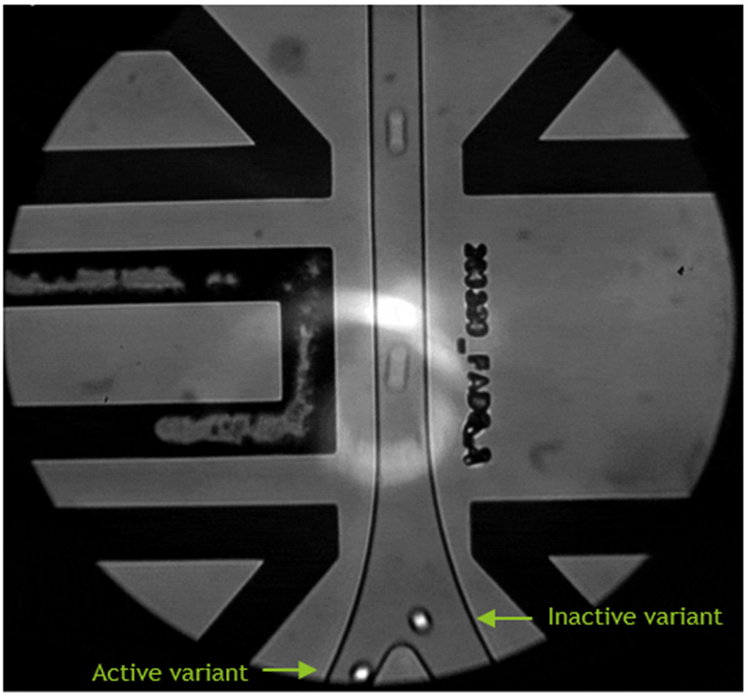
To make formaldehyde synthesis from methanol more sustainable, enzymes from three subclasses—dehydrogenases (NAD(P)+ or PQQ-dependent) and oxidases (FAD-dependent, oxygen-dependent)—were explored by TUM CBR and TUM EBT. Over 40 enzyme candidates were screened for methanol activity. Expressing oxidases (from eukaryotes) and PQQ-dependent dehydrogenase (from methanotrophs) in prokaryotic cells required effort but was successfully achieved for two eukaryotic oxidases for the first time.
Around 20 active candidates were characterized for methanol affinity and activity, stability, and overoxidation potential. NAD-dependent dehydrogenases showed low methanol affinity and activity and minimal overoxidation. They displayed moderate tolerance to methanol and low tolerance to formaldehyde, which is not surprising given its reactivity. Oxidases exhibited high methanol activity and affinity, but also significant formaldehyde overoxidation. PQQ-dependent dehydrogenase preferred ethanol, performing similarly to NAD-dependent dehydrogenases on methanol.
The representatives of each subclass of enzymes were wired with different electron mediators in the electrochemical reactor. After optimization, based on the best mediator-enzyme combination, the most suitable enzyme candidate will be selected for engineering. While high-throughput screening method and electrochemical reactor integration are in progress, rational enzyme engineering and immobilization strategies have been planned.